Fall 2025: COVID/flu/RSV Vaccine Availability! (Updated 1/7/26)
1/7/26: flu is ramping up up up. We still have flu shots available for all kids, privately insured adults, and uninsured adults. Schedule online or text us with questions (206-203-2509).
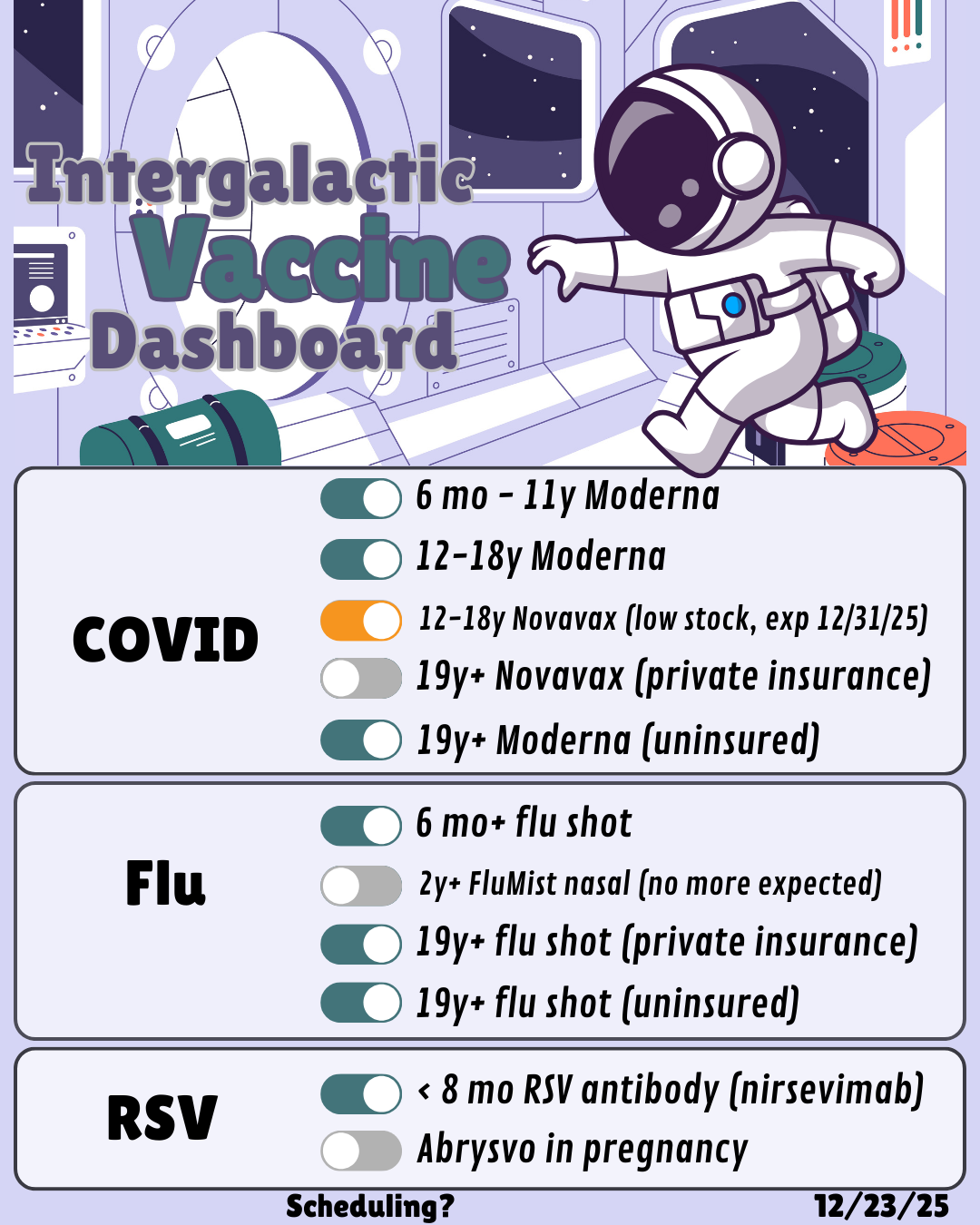
Dashboard updated 1/7/26:
Flu
Flu shots for babies 6 months and up: available!
FluMist nasal: out of stock, will not be able to reorder
Flu shots for adults with insurance: available!
Flu shots for uninsured/underinsured adults: available! limited stock, need to text office to verify eligibility (AVP Program)
COVID
Moderna for babies 6 months and up: in stock
Moderna for kids 12-18: in stock
Novavax for kids 12-18: out of stock
Novavax for adults: out of stock
Moderna for uninsured/underinsured adults: available! limited stock, need to text office to verify eligibility (AVP Program)
RSV (More about RSV products (PDF)):
nirsevimab (RSV antibody, brand name Beyfortus): in stock, schedule online to be given on/after October 1, and please include a best estimate on the baby’s weight (over or under 11 pounds). Please don’t pick a date before October 1 even though the scheduling module will have them available. Babies under 8 months are eligible, and some babies 8 months and up are eligible depending on risk factors.
If you’re 32-36 weeks pregnant, you can choose to receive Abrysvo RSV vaccine, or wait for baby to be born and receive nirsevimab. Our general preference is for baby to receive nirsevimab, since it’s a “sure thing” and seems somewhat more effective. We are not stocking Abrysvo this year since it’s readily available in the community.
Update 10/20/25 - we have multiple shipments arriving tomorrow - restocking Moderna for 6m—11y, Novavax for 12-18y, Novavax for adults.
Update 10/13/25 - we finally received shipping notifications today and should receive limited supplies of covid vaccines tomorrow 10/14! We’ve been reaching out to patients who have flu vaccines scheduled and will be opening up general scheduling later today.
Update 10/9/25 - we were told that our orders have gone in, so hopefully we’ll have covid vaccines for 6m-18y early next week. We’ll open up schedule as soon as we have a tracking number. Our Novavax order for adults is still in pre-order, somehow.
Update 10/6/25 - he finally signed off on the ACIP recommendations! Hopefully they’ll start shipping vaccines soon. As soon as we get tracking numbers we’ll open up scheduling.
Update 10/2/25 - you may have heard Dr Kass and a fabulous Intergalactic parent on NPR today talking about the holdup — the acting CDC director has not yet signed off on the ACIP recommendations, so the CDC can’t ship vaccines through the Vaccines for Children program. In Washington all our Childhood Vaccine Program vaccines come through this program, not just vaccines for kids with Medicaid. Pharmacies buy their vaccines through a different mechanism, which is why kids can get vaccinated at the pharmacy, but not in provider offices, but kids with Medicaid, and young children of any age, can’t get vaccinated in pharmacies :( We have no ETA on when the acting CDC director might do his part, but the state is exploring different mechanisms to procure the vaccines.
Update 9/29/25 - no update. No ETA on any of the COVID vaccines.
Update 9/19/25 - big day! We received Flumist and adult flu vaccine!
Update 9/18/25 - We have flu shots for babies/kids 6 months and up — schedule here! We don’t have Flumist (nasal vaccine) yet. Babies and kids through age 8 who haven’t already received two doses of flu vaccines before July 1, 2025 need two doses, 4 weeks apart.
We have nirsevimab (RSV antibody) on hand and can start giving it October 1 — schedule here and please include a best estimate on the baby’s weight (over or under 11 pounds). Please don’t pick a date before October 1 even though the scheduling module will have them available.
We are still waiting for CDC approval to order COVID vaccines and will keep updating this post with information on scheduling.
Lastly, on 9/17, the West Coast Health Alliance (WA, OR, CA, HI) issued recommendations for COVID-19, flu, and RSV vaccines.
Update 9/5/25 - Washington DOH issued a standing order that should allow anyone 6 months and up to get a vaccine without a prescription at any pharmacy (FAQ). Still, most pharmacies don’t immunize babies (though most will immunize kids 3 and up), we still don’t know about insurance coverage, and pediatric doses haven’t started shipping yet. We will continue updating this post (and our site) with availability.
Washington, Oregon, California (and now joined by Hawaii) have formed the West Coast Health Alliance — “The Alliance will help safeguard scientific expertise by ensuring that public health policies … are informed by trusted scientists, clinicians, and other public health leaders. [The states] will start coordinating health guidelines by aligning immunization recommendations informed by respected national medical organizations. This will allow residents to receive consistent, science-based recommendations they can rely on — regardless of shifting federal actions.”
We were directed to stop giving 24-25 vaccines, as the FDA terminated the emergency use authorizations and delisted last year’s approved vaccines, so we do not have any COVID vaccines at all right now. Ordering has not yet started for the updated vaccines.
Original post:
We are in a tricky spot. COVID cases are rising. Schools are about to start. We all have lots of questions about COVID vaccine – will my child be eligible for a booster dose? For an updated vaccine? Will insurance cover it?
Though I typically like to make recommendations based on solid data, most of what we have right now is conjecture. I might be wrong, things might change, but this is the best I’ve got.
Given the AAP’s recommendations (discussed below), and the state’s commitment to immunization, I anticipate that we will have updated COVID vaccines for everyone 6 months and up.
We do not know when they will ship. Last year we didn’t get them until late September.
We do currently still have a limited supply of 24-25 COVID vaccine available for self-schedule. We are working on sourcing more.
As clinical, evidence-based recommendations diverge from CDC recommendations, it becomes more difficult to answer more technical questions, like, if your child gets a booster now, can they get an updated booster later in the season? We don’t even have updated 25-26 recommendations from the CDC yet, but the 24-25 recommendations emphasize staying up to date and getting the updated vaccine. Presumably if your child got a 24-25 dose now, they would still be eligible for a 25-26 dose later in the season (but I can’t guarantee it and have no idea whether your insurance company will agree). The concept of ‘eligible’ becomes less clearly defined since the CDC now recommends shared decision making (“Parents of children ages 6 months to 17 years should discuss the benefits of vaccination with a healthcare provider”) and the AAP “recommends the vaccine be available for children ages 2-18 who do not fall into [high] risk groups, but whose parent or guardian desires them to have the protection of the vaccine.” (Babies 6—23 months are considered high risk by the AAP.) That should mean that if you want your child vaccinated, you should be able to get them vaccinated. I can imagine that without a blanket recommendation, it might be more difficult to access vaccinations in pharmacies or events that rely on “standing orders” (versus an encounter with a provider). I am not anticipating this to be a barrier for us in our clinic.
Here are the challenges:
- The FDA approves and ‘labels’ vaccines – labels say for what and whom the vaccine is for. In the case of Moderna’s vaccine, when it went from being authorized for emergency use (EUA) to getting full approval, it was approved for people who are “65 years of age and older, or, 6 months through 64 years of age with at least one underlying condition that puts them at high risk for severe outcomes from COVID-19.” This change was not recommended by agency experts, but instead was put in place by the political appointee heading the FDA at the time. Vaccines can be given off-label but it’s not clear what that would entail in today’s medico-political environment.
- We know that babies under 2 are at very high risk of complications from COVID-19, and that half of young people with complications did not have underlying health conditions.
- COVID causes severe disease in babies under 6 months. These babies cannot receive vaccine directly, so they are relying on transplacental antibodies produced by their parent during pregnancy. The CDC removed the recommendation for all pregnant people to receive COVID vaccines, despite evidence strongly supporting the safety and efficacy of these vaccines. The AAP, ACOG and dozens of other professional organizations strongly disagreed, and continue to urgent vaccination during pregnancy. On August 22, 2025, ACOG released updated clinical guidance on immunization for influenza, COVID and RSV in pregnancy, reiterating strong recommendations.
- There are rumors that the FDA might not extend the emergency use authorization of Pfizer’s pediatric COVID vaccine. While we don’t carry the Pfizer COVID vaccines, if this happens, it would likely impact availability of Moderna, since Moderna would need to significantly increase production to meet demand. Additionally, Pfizer’s vaccine was still on EUA and was recommended for all children 6 months and up, without the FDA restriction of having an underlying condition. It is inconceivable that we are more than halfway through August and don’t yet know if there will be a Pfizer vaccine available.
As the CDC and FDA have been undermined by political appointees, professional and academic organizations have stepped up to provide informed, evidence-based recommendations. On August 19, 2025, the Vaccine Integrity Project presented a detailed literature review exploring the current state of the evidence on immunization for COVID-19, RSV and influenza – the recording and slides are available on their website. It is clear from the literature that COVID continues to pose a significant risk to infants and young children, and that all people deserve to have their first encounter with COVID be with a vaccine, not the virus itself.
Also on August 19, 2025, the AAP released their own immunization schedule and recommendations.
COVID-19 continues to result in hospitalization and death in the pediatric population. Infants and children 6 through 23 months of age are at the highest risk for severe COVID-19. Given this, the AAP recommends a COVID-19 vaccine for all children ages 6 through 23 months old to help protect against serious illness. Children younger than 2 years old are especially vulnerable to severe COVID-19 and should be prioritized for vaccination unless they have a known allergy to the vaccine or its ingredients.
In addition to the recommendation for all children younger than 2 years, the AAP recommends a single dose of age-appropriate COVID-19 vaccine for all children and adolescents 2 through 18 years of age in the following risk groups :
· Persons at high risk of severe COVID-19
· Residents of long-term care facilities or other congregate settings
· Persons who have never been vaccinated against COVID-19
· Persons whose household contacts are at high risk for severe COVID-19
The AAP also recommends the vaccine be available for children ages 2-18 who do not fall into these risk groups, but whose parent or guardian desires them to have the protection of the vaccine.
Given the AAP’s recommendations (discussed above), and the state’s commitment to immunization, I anticipate that we will have updated COVID vaccines for everyone 6 months and up.
We plan to stock Moderna pediatric vaccines, Novavax for 12—17-year-olds, and private pay Novavax for adults. We also plan to stock flu vaccines (pediatric and private pay adult), RSV antibody for young infants, and Abrysvo RSV vaccine for people in pregnancy.